Our solution is simple, overcome the issues currently facing clinical trials including patient accrual, timelines, budgets, and quality of data.
- Conduct clinical trials in the untapped Belarus market to reduce the overall time and cost of clinical trials by > 50%, while generating high quality data
- As a US based company, we use the same high standards used to conduct clinical trials in the US
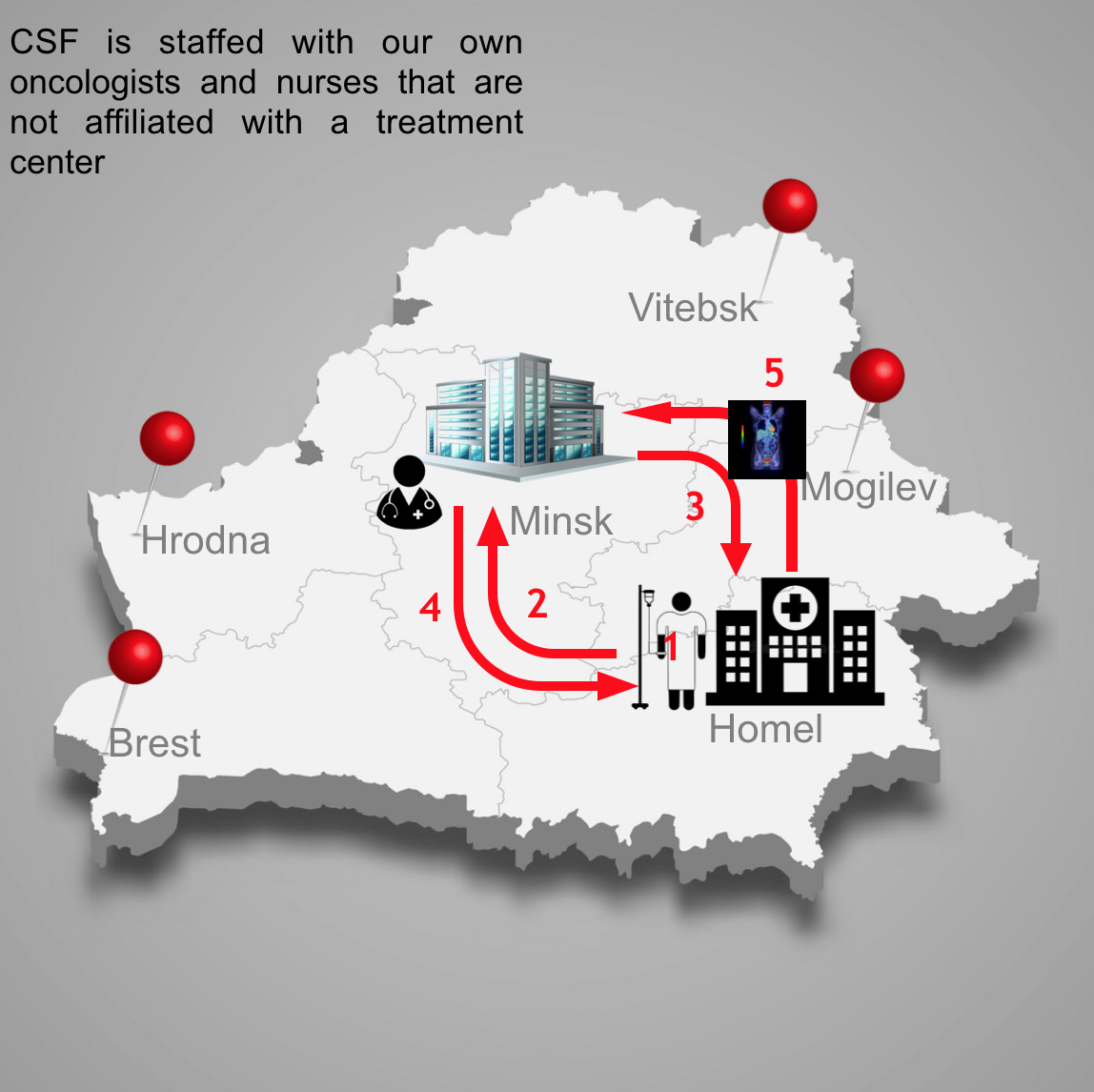
- Our novel Centralized Screening Facility allows us to provide the highest quality data by ensuring patients meet all trial eligibility criteria
- Able to have first patient screened within 120 days of protocol submission to health authorities (Average is 90 days)